"establishing by objective evidence process control limits and action levels which result in product that meets all predetermined requirements."
Number of Worst Cases
Process OQ vs. Equipment OQ
Why Should You Perform an Operational Qualification (OQ)?
- Identify critical parameters, characterize the process, and establish an operating window (also referred to as a process window).
- Run the process under worst-case conditions to demonstrate that these conditions result in an acceptable product; this further demonstrates that the process is robust against variation of process parameters [1].
Operational Qualification Protocol
Why Test Method Validation Must Come Before Operational Qualification
How to Establish a Process Window for Operational Qualification
- Software parameters
- Raw material specification
- Process operating procedure
- Material handling requirements
- Process change control
- Training
- Environmental conditions
Operational Qualification (OQ): Running at Worst-Case Settings and Acceptance Criteria
- Temperature (T): 150,0 ± 10,0 °C
- Time (t): 2,0 ± 0,5 sec
- Pressure (p): 3,0 ± 0,5 bar
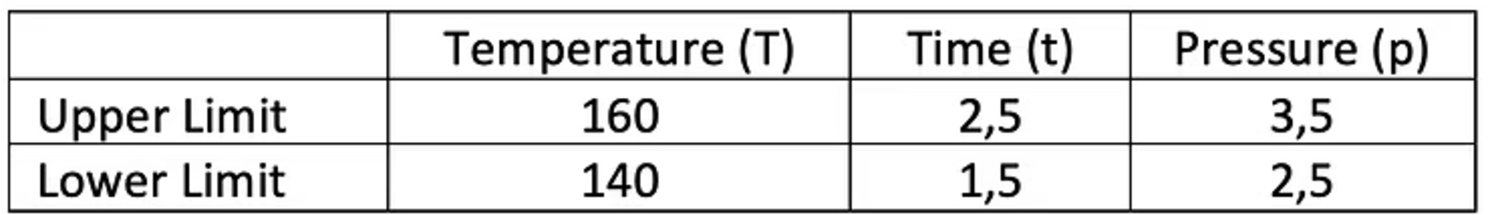
Table 2 – Worst-Case Parameter Settings
Now you know what parameters you must set to run the Operational Qualification studies.
To answer the question of how many samples you must produce, read our blog posts about "Process Validation Sample Size“ and “Statistical Tolerance Interval".
A tip related to sample size and Operational Qualification is that you can use reduced reliability levels (with a confidence level of at least 95%) for OQ runs.
What Do We Mean by "Worst-Case"?
Acceptance Criteria for Operational Qualification
Analyzing Operational Qualification Results
Best Practices for Operational Qualification
Common Challenges in Operational Qualification
Further helpful links & resources:
- SIFo Medical YouTube Channel: Short, valuable videos on Quality Management.
- Free Resources: Get free access to checklists & templates.
- TMV Guide: Your practical guide to perform test method validation (incl. templates & videos).
- TMV Online Course: Become an expert in Test Method Validation
- SIFo Medical Newsletter: Join our mailing list to receive updates and exclusive content delivered straight to your inbox as soon as it's released.
Are you unsure how to perform an Operational Qualification (OQ)? Contact us today at office@sifo-medical.com, and we'll support you perform an OQ correctly.
References
[1] W. Taylor, Statistical Procedures for the Medical Device Industry. Taylor Enterprises, Inc., 2017. [Online]. Available: www.variation.com
[2] Global Harmonization Task Force, "Quality Management System - Process Validation Guidance," GHTF/SG3/N99-10:2004, 2004. [Online]. Available: http://www.imdrf.org/docs/ghtf/final/sg3/technical-docs/ghtf-sg3-n99-10-2004-qms-process-guidance-04010.pdf
[3] Quality-on-site.com, "GMP Documentation: Process Validation," [Online]. Available: http://www.quality-on-site.com/get.php?fileid=139
[4] ISO 11607-2:2019, Packaging for terminally sterilized medical devices — Part 2: Validation requirements for forming, sealing and assembly processes. International Organization for Standardization, Geneva, Switzerland, 2019.
[5] ISO 13485:2016, Medical devices – Quality management systems – Requirements for regulatory purposes. International Organization for Standardization, Geneva, Switzerland, 2016.
[6] U.S. Food and Drug Administration, "21 CFR Part 820 - Quality System Regulation," [Online]. Available: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=820&showFR=1

